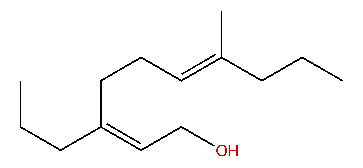
| (Z,E)-7-Methyl-3-propyl-2,6-decadien-1-ol |
| Formula: | C14H26O |
| CAS#: | 38789-38-7 |
| MW: | 210.36 |
[MS]
[ NMR ]
[Behavioural function]
[Chemdraw]
|
|
Reference(s) for synthesis of (Z,E)-7-Methyl-3-propyl-2,6-decadien-1-ol
| Bowlus, S.B., and Katzenellenbogen, J.A. 1973. A highly stereoselective synthesis of (2Z,6E)-7-methyl-3-propyl-2,6-decadien-1-ol. A proposed revision in the stereochemistry of a tetrahomoterpene isolated from the codling moth. Tetrahedron Lett. 14:1277-1280. |
| |
| Bowlus, S.B., and Katzenellenbogen, J.A. 1973. Synthesis of the natural isomer of a tetrahomoterpene alcohol obtained from the codling moth. J. Org. Chem. 38:2733-2734. |
| |
| Cooke, M.P., Jr. 1973. A stereoselective synthesis of a proposed codling moth sex pheromone. Tetrahedron Lett. 14:1281-1284. |
| |
| Cooke, M.P., Jr. 1973. Synthetic proof of the structure of a proposed codling moth sex pheromone. Tetrahedron Lett. 14:1983-1986. |
| |
| Kovalev, G.G., Khusid, A.Kh., Konyukhov, V.P., Nedopekina, S.F., and Neimark, Ya.L. 1975. Synthetic investigations in the field of sex attractants of insects. II. Synthesis of a mixture of the geometric isomers of 7-methyl-3-propyldeca-2,6-dien-1-ol, one of the components of the sex pheromone of the codling moth. Zhur. Org. Khim. 11:1183. |
| |
| McDonough, L.M., George, D.A., Butt, B.A., Ruth, J.M., and Hill, K.R. 1972. Sex pheromone of the codling moth: structure and synthesis. Science. 177:177-178. |
| |
| Morizur, J.-P., Muzard, G., Basselier, J.-J., and Kossanyi, J. 1975. Chimie des insects. II. Synthese du methyl-7-n-propyl-3-decadien-2(Z),6(Z)-ol-1 isole chez Laspeyresia (Carpocapsa) pomonella. Bull. Soc. Chim. Fr. 43:257. |
| |
| Obayashi, M., Utimoto, K., and Nozaki, H. 1977. Magnesium iodide induced rearrangment of alpha,ß-epoxysilanes to ß-ketosilanes as applied to the stereoselective synthesis of tetrahomoterpenoid of codling moth. Tetrahedron Lett. 18:1807-1809. |
| |
| Ouannes, C., and Langlois, Y. 1975. Nouvelle synthese stereoselective d'un tetrahomoterpene extrait de Laspeyresia pomonella L. et de ses trois isomeres Geometriques. Tetrahedron Lett. 16:3461-3464. |
| |
| Rossi, R. 1977. Insect pheromones. I. Synthesis of achiral components of insect pheromones. Synthesis. 12:817-836. |
| |
|







