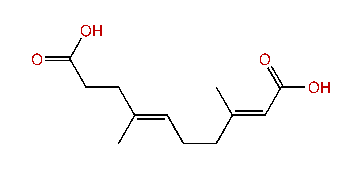
| (E,E)-3,7-Dimethyl-2,6-decadien-1,10-dioic acid |
| Formula: | C12H18O4 |
| CAS#: | 22264-07-9 |
| MW: | 226.27 |
[MS]
[ NMR ]
[Behavioural function]
[Chemdraw]
|
|
Reference(s) for synthesis of (E,E)-3,7-Dimethyl-2,6-decadien-1,10-dioic acid
| Meinwald, J., Chalmers, A.M., Pliske, T.E., and Eisner, T. 1969. Identification and synthesis of trans, trans-3,7-dimethyl-2,6-decadien-1,10-dioic acid, a component of the pheromonal secretion of the male Monarch butterfly. J. Chem. Soc. Chem. Comm. 3:86-87. |
| |
| Miles, D.H., Loew, P., Johnson, W.S., Kluge, A.F., and Meinwald, J. 1972. A short stereoselective synthesis of some terpenes from the pheromonal secretion of the queen and Monarch butterflies. Tetrahedron Lett. 30:3019-3022. |
| |
| Ono, N., Hamamoto, I., and Kaji, A. 1985. Terpenoid synthesis via palladium-catalyzed allylic alkylation of allylic nitro compounds. Synthesis. 10:950-952. |
| |
| Trost, B.M., Bogdanowicz, M.J., Frazee, W.J., and Salzmann, T.N. 1978. Oxasecoalkylation via cyclobutanone intermediates. J. Am. Chem. Soc. 100:5512-5525. |
| |
| Tsuji, J., Kataoka, H., and Kobayashi, Y. 1981. Regioselective 1,4-addition of nucleophiles to 1,3-diene monoepoxides catalyzed by palladium complexes. Tetrahedron Lett. 22:2575-2578. |
| |
|







